Can anyone with some solid knowledge on this topic let me know if this is safe/unsafe? It’s ink, that is cured with a UV light. I would be printing, then lasering after the ink is cured. I assume it’s a no because I see the word “vinyl”, but i’m no chemist. Thanks!
With vinyl, you’re worried about PVC, the chloride is the problem. Not all vinyl compounds have a chloride, looks like this one doesn’t have it.
Do they have a section about combustion products? That’s where I’d be focusing my attention.
How much are you thinking will be burned? There is a point where something like ink may have so small a volume that it isn’t harmful unless you are doing all day long every day for months.
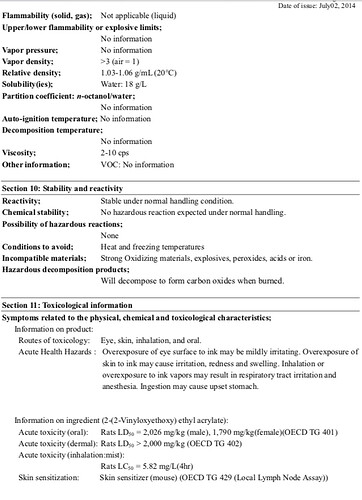
The decomposition products do not look harmful to me - Carbon oxides are pretty much CO and CO2, basic burning results for most materials.
I’d need to be cutting through it mostly all the way around the object—like through a “bleed” area on the print. I suppose I could go a tiny bit outside of the printed area to be safe, it’s just hard with the less than accurate laser alignment sometimes.
I asked the ink company if it was safe to laser and they just said, “here’s the safety data sheet, you can make the call”. Not exactly helpful of them haha!
You may want to do a burn test. There are several links in this forum and online on how to do a safety burn test.
Basically what @ben1 said: carbon oxides… I wouldn’t worry much there. And you really won’t be burning much, so I would really not worry. Your risk profile is up to you, of course.
You have the MDS for the ink, not the ink after it is cured. The UV light does something to its chemical structure. The laser will combust (most likely) the cured ink. This may make a difference (good or bad) or it may not.
The vinyl you see, according to pubchem via google, has a molecular formula of C9H14O4 - no chloride, just carbon, oxygen and hydrogen. So as evansd2 said, it seems to be okay.
First it is important to understand that MSDS or SDS are written by lawyers mostly for lawyers and apply mostly to industrial scales of materials. They are not usually a good source of material properties, but may be the only source that is readily available. This one does cite toxicity data for the primary component, which does say something, but at 2 g/kg in rats that is not very toxic. They are probably most useful to first responders to chemical sites with large quantities.
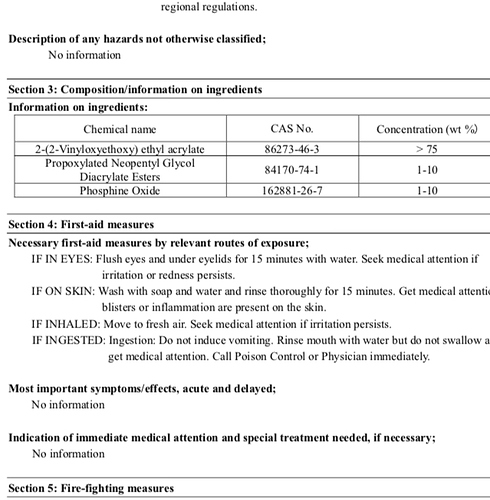
The phosphine oxide on the MSDS is actually Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide which can be determined from the CAS number.
It a photo-initiator. it is probably more towards 1% total than 10%. It will absorb UV photons and form free radicals. The free radicals will then activate the vinyl groups on the 1-[2-(Vinyloxy)ethoxy]ethyl acrylate and diacrylate ester. The activated vinyl radical will then react with another vinyl, i.e. polymerize. Without the photoinitiator, the UV activation would be slow. The end product will be a polymer quite similar to polymethyl methacrylate (PMMA) more commonly known as acrylic. Laser ablation of the ink would probably have an odor similar to acrylic, but the mass of the ink being exposed to the laser is probably quite small. Standard safety precautions for the laser cutter vent should suffice. Colored inorganic pigments could also be released, but again the amount would be minuscule.
Jeff Forbes, Ph.D. (Physical Chemistry)
Yep, that’s exactly what I was about to say.

You guys are awesome! Thank you so much for answering, I appreciate it!